Lubricent® UV
UV Curable Hydrophilic Coatings
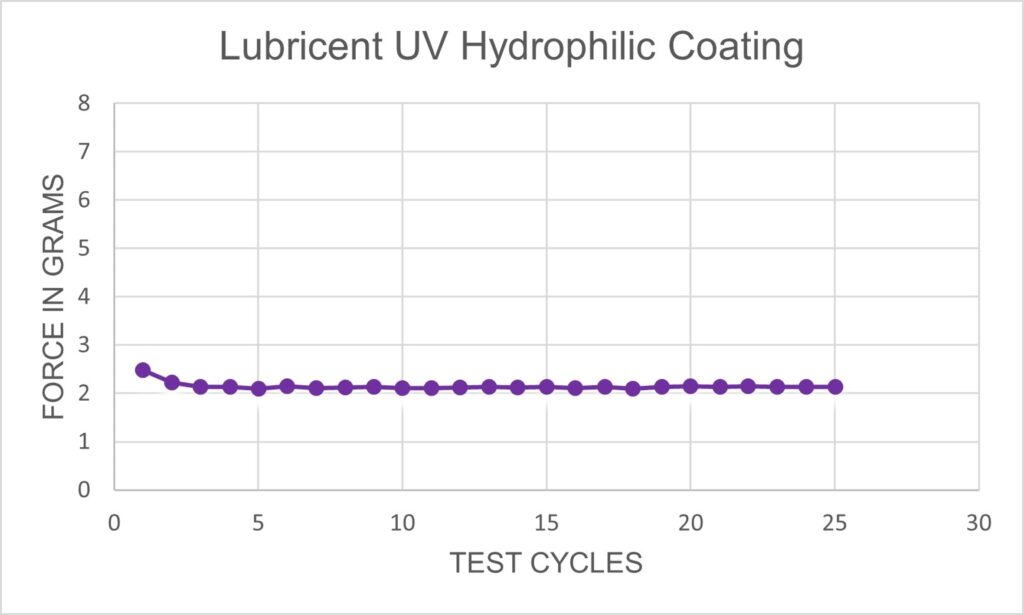
Harland’s Lubricent UV hydrophilic coatings provide exceptional friction reduction with state-of-the-art durability. Lubricent coatings enable catheters, sheaths, introducers, guide wires and other medical devices to navigate through the most difficult, tortuous vessels and anatomies. The solution blends a hydrogel-forming polymer with a proprietary photo-reactive compound dissolved in isopropyl or ethyl alcohol. With Lubricent UV coating on your device, you’ll get best in class lubricity and durability no matter the medical application.